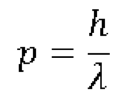(#6) Heisenberg's Uncertainty Principle - certainly taught wrong
Heisenberg's principle shone new light on the Quantum world and may hold the keys to the mystery of our origins
One of the fundamental principles in the Quantum physics was put forward by German Physicist Werner Heisenberg in 1927. The principle came to be known as ‘Heisenberg’s Uncertainty Principle’ denoted by below equation:
(an unrelated trivia: Heisenberg almost failed to defend his PhD thesis before the committee was nudged by his mentor Sommerfeld to grant him PhD; he just managed to get doctorate with the lowest possible grade. Read more here).
The principle states:
both the position and momentum of an entity cannot be determined precisely simultaneously. The precision in determination of one quantity (position or momentum) will introduce the imprecision (uncertainty) in the determination of another quantity (position or momentum).
This principle is highly non-intuitive and for the most part taught incorrectly in schools and on various social media. So I am going to try my bit to explain it as best as I could.
Before getting ahead of ourselves, lets look at the wave-particle duality principle. Back in nineteenth century Maxwell and others had proved that light is a wave (you can read more about this in my post here). In early twentieth century Einstein built on the work done by Max Planck and proved that light is a particle.
Einstein went on to postulate Photoelectric Theory and was awarded a Nobel Prize for this work.
It was established by 1920’s through experimentations as well that light indeed is both a wave and a particle.
Well so where does this new revelation leaves us.
The boundaries of particle and wave nature of matter was blurring faster than the speed of light in the earlier part of twentieth century.
Werner Heisenberg working closely with Pauli Wolfgang, Max Born, Neils Bohr and others came up with the mathematical formulation of Uncertainty Principle. It was a rather non-intuitive yet a fundamental principle which took sometime for folks to wrap their heads around.
You may ask - how can you not be able to determine both position and momentum of something precisely? We do that on daily basis - e.x. when you are driving in a car, would you not know your position relative to an arbitrary chosen origin, or momentum or the direction of movement. This principle doesn’t make sense when you look around yourself in a non-quantum world.
Precisely why this is a rather non-intuitive principle - well to be fair most of the quantum physics is in fact non-intuitive.
So, how did modern folks taught and explained this non-intuitive principle to incoming generations of physics enthusiasts.
By either dumbing this principle down to a simplistic and incorrect formulation or just by remaining ignorant themselves of the actual meaning and far reaching implications of this principle.
Don’t take my word for it - checkout these two prominent, famous and high quality channels explaining this principle to their audience of millions:
So you ask, what is incorrect with the explanations? Well almost everything!!!
What they described above for majority of time in the video is ‘Observer Effect’
Observer effect is the disturbance of an observed system by the act of observation
Per folks above there is uncertainty in the position and momentum quantities because we cannot ‘measure’ due to the miniature nature of the quantum world and light photons.
Well our inability to not be able to measure do not warrant a law or principle - that warrants more precise instrumentation. Isn’t it?
Lets try to understand this principle by experimenting:
Momentum of a wave is defined in terms of its wavelength given by below formula:
Lets try to measure momentum and position both with certainty and see where we land below:
Measuring Momentum with Certainty
So now armed with information above we can determine the momentum of the below uniform wave accurately:
Remember that the wave is the ‘trajectory’ of a particle. The particle can be anywhere on this entire wave trajectory. So do you know where the particle is at any arbitrary time on this infinite long wave trajectory. NO we don’t - particle has equal probability to be anywhere on this infinite long wave trajectory.
So we conclude that although we know the momentum with certainty but not the position.
Measuring Position with Certainty
Lets flip the situation and start from determining the position accurately.
How do we determine accurately where the particle is?
Well, by localizing the particle to a small area, by changing the probability density. Instead of being comfortable with the equal probability of finding the particle at any point on this wave - lets try to localize this particle to a smaller and smaller area.
(analogy: imagine as a parent, instructing your kid to ONLY play in a designated area lets say - park. Essentially what you have done is almost accurately determined the position of your kid in the park when you go out to collect your kid).
So, we bring in more waves and introduce the interference between the waves in such a way that the resultant wave is more localized increasing the probability of finding the particle at a specific position thereby enabling us to accurately measure the position of the particle.
What about momentum? Well, we lost it. Remember we introduced interference in the above picture by bringing in multiple waves - well those waves had different wavelengths, and hence different momentums.
I do not know now what the resultant wave’s momentum is.
So we conclude that the accurate determination of position forced us to abandon the pursuit to determine the accurate momentum of particle.
This is how the certainty in the determination of one complimentary variable (position and momentum are complimentary variables as are Energy and time and bunch more) introduce the uncertainty in the determination of another variable.
Well, before I sign off - you may be wondering what is the implication of this principle. A fact such as Quantum Tunneling (a phenomena that a wave can pass through a potential barrier even if the wave has no energy to do so) is well explained by Heisenberg’s Uncertainty Principle.
Without Quantum Tunneling, life itself and very little else, would exist. Tunneling allows protons in the core of the sun to overcome mutual repulsion caused by their positive charges, a potential barrier that they do not have the kinetic energy to overcome. This allows the formation of Deuterium and begins the nuclear fusion process in the star’s core which leads to the formation of helium from hydrogen. All a star’s energy output is, by extension, a result of aspect of nature described by the Heisenberg uncertainty principle. Lets go further than this.
This fantastic aspect of nature also allows for the creation of something from almost literally nothing and that may hold the keys to the mystery of our origin in the universe.
=====================================================================
Sources:
http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/barr.html
https://sciscomedia.co.uk/heisenberg-uncertainty-principle-a-familiar-concept-misunderstood/
https://en.wikipedia.org/wiki/Uncertainty_principle